Our recent research highlights the development of a mesoporous polymer separator tailored for lithium metal batteries (LMBs), which promises to address crucial safety and performance challenges. Published in Energy Storage Materials under the title “Nanoporous Polymeric Membranes with Tunable 10-nm Pore Sizes for Fast Li+ Ion Mobility and Enhanced Cyclability in Lithium Metal Batteries” by Taeseok Oh, Rak Hyeon Choi, Hye Ryung Byon, and Myungeun Seo, this study reveals innovative strategies to improve Li+ transport and stability within these high-energy-density batteries (see the full paper here). Taeseok and Rak Hyeon were co-first authors of the paper responsible for the membrane development and the electrochemical study, respectively.
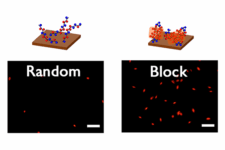
Lithium metal batteries are poised to meet increasing energy density demands; however, the formation of lithium dendrites and inactive lithium during charge and discharge cycles has raised significant safety concerns. We focused on controlling pore sizes within the mesopore region to strike a balance between ion conductivity and selectivity—an area often overlooked in existing separators, which predominantly feature macropores or micropores.
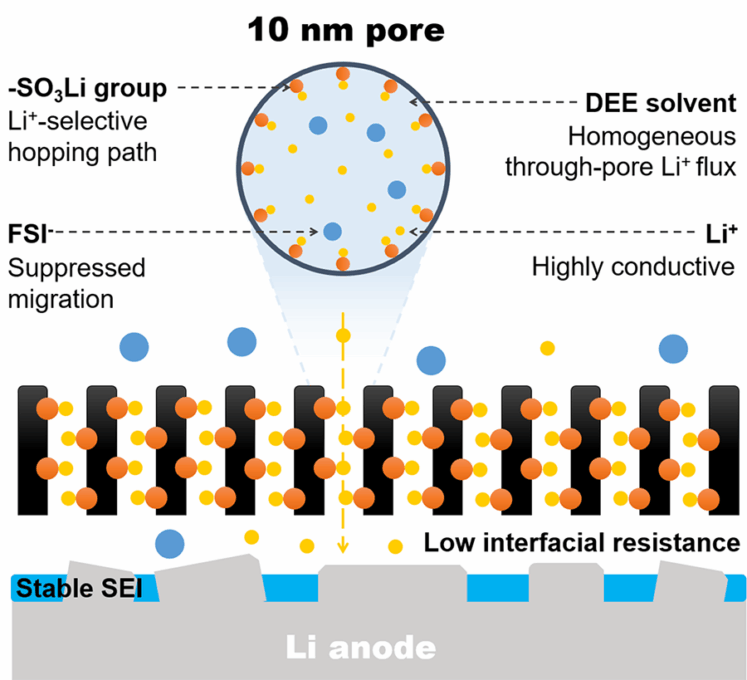
Based on our unique polymerization-induced microphase separation (PIMS) approach, which enables the production of robust mesoporous membranes with a 3D continuous pore structure, we further optimized the membrane fabrication process to increase the cross-linking density of the membranes, allowing them to maintain their integrity under high temperatures and in organic solvents. Subsequent sulfonation immobilized sulfonic groups on the pore surface, as we previously reported (see our ACS Appl. Energy Mater. paper here). By exploiting the advantage of the straightforward pore size control provided by PIMS, we discovered a direct correlation between pore size and ionic conductivity through the membrane. While the conductivity increased with the increasing pore size in mesoporous membranes without sulfonation, the sulfonated membranes showed much higher conductivities that peaked at 10-nm pores. Combined with the higher Li+ transference numbers and lower activation energy for the conduction, the results indicate that sulfonic acid groups significantly boost Li+ transport while simultaneously inhibiting anion conduction.
The uniform Li+ flux through 10-nm pores led to improved Li+ mobility, reduced interfacial resistance, and suppressed anion transport compared to commercial separators. When the membrane was included in Li|LiFePO4 full cells with an N/P ratio of 2.3, the separator showcased robust cycling stability over 1000 cycles, indicating significant potential for practical applications.